
What is central sleep apnea?
Central Sleep Apnea
Excessive daytime sleepiness, a lack of energy during the day, or pauses in breathing at night may all be symptoms of a serious sleep disorder called central sleep apnea (CSA).
What is central sleep apnea?
Most people associate “sleep apnea” with a specific sleep disorder called obstructive sleep apnea (OSA).
Although symptoms of OSA and CSA are similar, they have different causes:
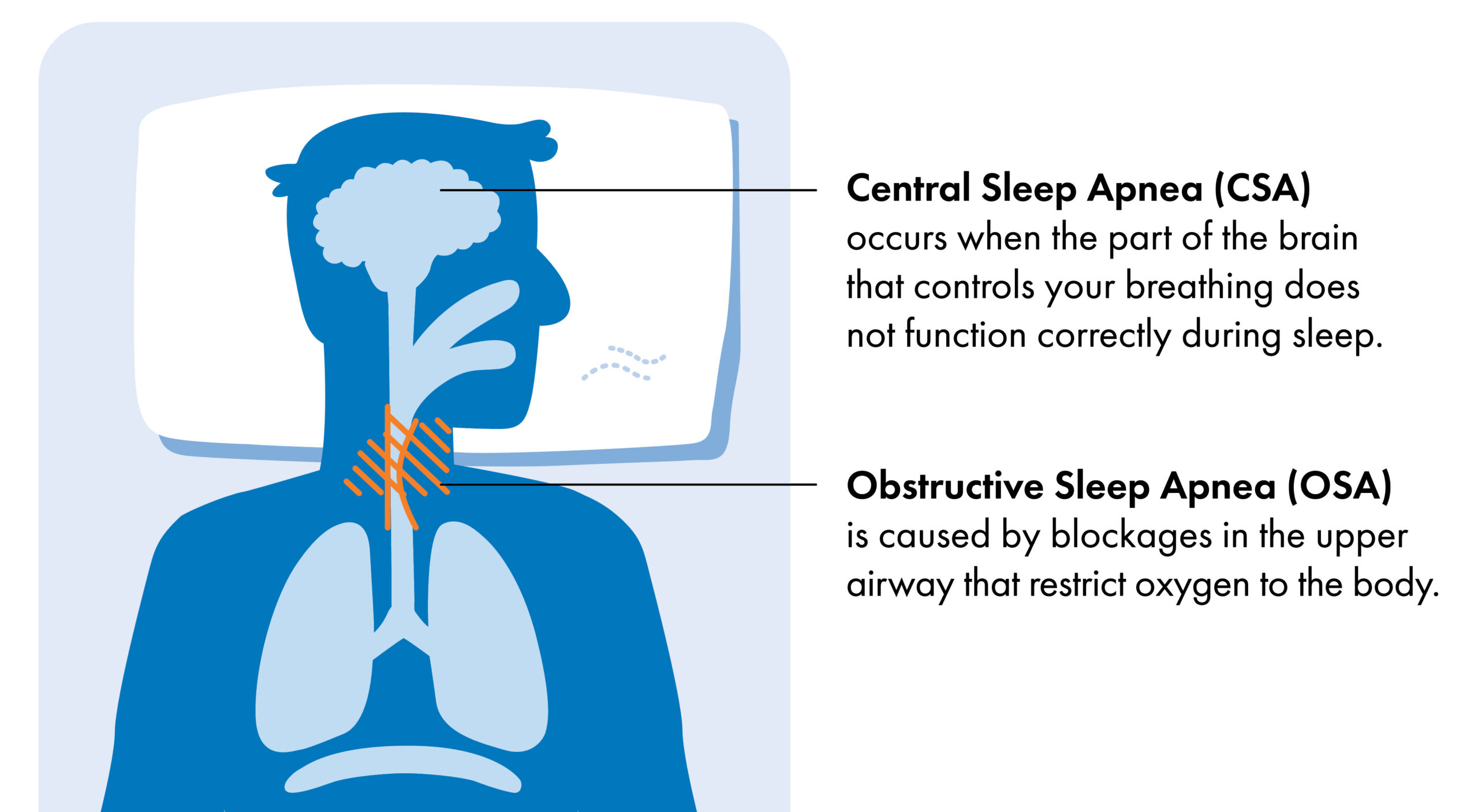
- In OSA, breathing is obstructed by a narrowing or blockage of the airway often caused by a person’s anatomy, or by relaxation of the chest and neck muscles during sleep.
- In CSA, the brain does not properly signal the body to stimulate a regular breathing pattern.1 While CSA is a less common and less studied form of sleep apnea, it’s just as serious as OSA.
What are the symptoms of CSA?
If left untreated, central sleep apnea can cause:2,3

Chronic fatigue

Excessive daytime sleepiness

Brain fog or cognitive impairment

Difficulty falling asleep or getting restful sleep
Many patients with CSA also have heart disease, especially heart failure.4 Within this population, patients with CSA are at increased risk for hospitalizations and even death.5,6
What causes CSA?
Central sleep apnea has several known causes or associations, including:
- Cardiac disorders, including congestive heart failure and atrial fibrillation (AFib)1
- High altitude1
- Opioids1
- Use of Positive Airway Pressure (PAP) therapy, such as CPAP or BiPAP1
- Idiopathic, or unknown1
How is CSA diagnosed?
Sleep apnea is diagnosed through a sleep study prescribed by a physician.
There are two main types of sleep studies:

Home Sleep Apnea Test
(Also called HSAT, Polygram or PG)
Completed at home
Measures oxygen, air flow and movement of the chest and abdomen*

In-Lab Sleep Study
(Also called Polysomnogram or PSG)
Completed overnight in a sleep lab
Assesses sleep apnea, sleep stage and other sleep disorders
During the sleep study, the number of apnea and hypopnea events per hour are counted and reported as the Apnea Hypopnea Index (AHI), the most common measure of severity of sleep apnea. Only a physician can diagnose whether you have sleep apnea, and whether your primary type of sleep apnea is central or obstructive.

